With experience acquired since 2011 in the cosmetic industry, Edencos guides you all along your products lifecycle, behind the scene formulation, regulatory process production.
Cosmetologist, expert in Natural Cosmetic, Edencos guides you in your strategic choices, with a panoramic view on cosmetic formulation, whether it is conventional, natural or certified.
Partnership, Creative freedom and Efficiency
Each client is unique in its choices and needs, that’s why Edencos is your custom-made partner to Imagine and Accomplish your projects.
5 Expertise centers at your service to move forward together into tomorrow’s cosmetic:
R&D – Regulatory Affairs – Project Management – Product Safety Evaluation – Quality – Audit
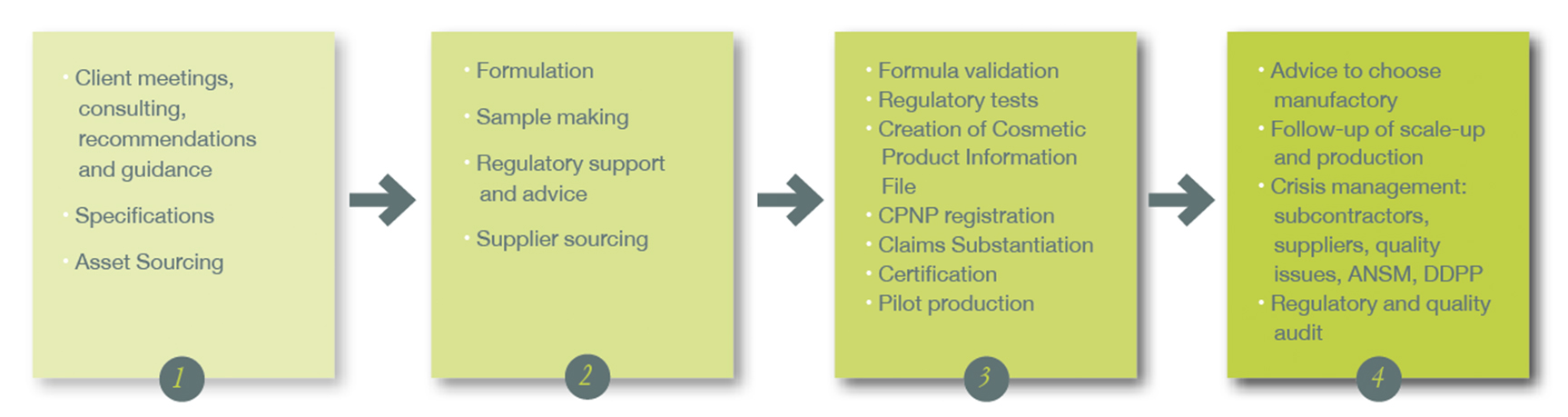
Edencos takes action at every step of the product lifecycle.
Research and development: innovative formulas
We provide you with a large portfolio of formulas, allowing us to answer all your needs in face care, body care, hygiene and hair care. From standard formula to custom-made product, reactivity and creativity are the driving forces of our R&D. Edencos is commited to respecting the formulation cost related to your marketing brief.
We accompany you in your products development with confidentiality, rigor, transparency and trust. We also achieve the validation tests needed for creating your cosmetic file.
- Raw materials or standard formulas sourcing
- Objectified actives sourcing
- Competitive intelligence, new products
- Formulation, sample making
- Stability and compatibility tests
- Evaluation of the PAO (Period after Opening) and/or Date of Minimum Durability
- Challenge Test, Tolerance tests, Efficacy tests
- Driving of industrial scale-up
- Pilot batch completion
Our mission: follow regulatory evolutions and inform you.
We help you understanding the legal documents so as to anticipate, achieve and update your formulas and product information files.
Competitive intelligence:
- Follow-up of international and European regulations
- Control of product claims, labelling and communication
- Proposition of claims adapted to your efficacy tests
- Validation of regulatory conformity for raw materials and formulas.
Creation of the Product Information File (PIF)
- Description of product
- Parts A and B of safety report
- Description of production method and GMP compliance statement
- Justifications of the claimed effects
- Data regarding animal testing
Registration
- Creation of your organization on European portal (CPNP)
- Products registration on portal
- Entry and validation of ingredients, formulas, labels, and packaging with Ecocert or Qualité France
Consulting, technical and legal support
- Writing and establishing subcontractors’ contracts
- Designating Edencos as responsible person
- EU domiciliation for foreign companies
- Guidance and interaction with competent authorities (DDPP, DGCCRF, ANSM) during controls
- Crisis management
- PIF auditing
Project management: A step-by-step visibility
Efficient and responsive, Edencos provides a dynamic and flexible development structure to achieve your projects while meeting deadlines.
- Consulting
- Retro planning creation
- Priorities and risks management
- Sourcing and supplying of raw materials
- Cost optimization
- Research and recommendation of industrial partners adapted to your needs in development and production
- Industrial guidance
Toxicological product evaluation: beauty with complete safety
If cosmetic products are not subject to prior authorization before entering the market, they need to be safe for human health. In order to ensure their safety, the European regulation enforces a toxicological evaluation of the cosmetic product and its ingredients. Edencos handles and validates for you part A and B of the cosmetic product’s safety report.
- Realization and evaluation of cosmetic product’s stabilities and compatibilities
- Microbiological quality check
- Research, analysis and review of impurities and traces in raw materials
- Definition of the exposure scenario for your cosmetic product
- Calculation of exposure values – SED
- Bibliographic research and toxicological profile creation for substances and mixtures
- Margin of safety calculation – MOS
- Writing and validation of part A
- Writing and signing of part B
- Cosmetovigilance monitoring
Audit and certification: quality the easy way
- Dossiers follow-up and audit management with certifying organizations (Ecocert, Qualité France etc.) and associations (Cosmebio)
- Follow-up and audits of subcontractors, production sites, etc.
- Global managing of quality
- Creation and implementation of quality procedures adapted to your structure